Clinical Trial Opens to Study Groundbreaking 3D Printed Device for Babies with Rare Respiratory Disease
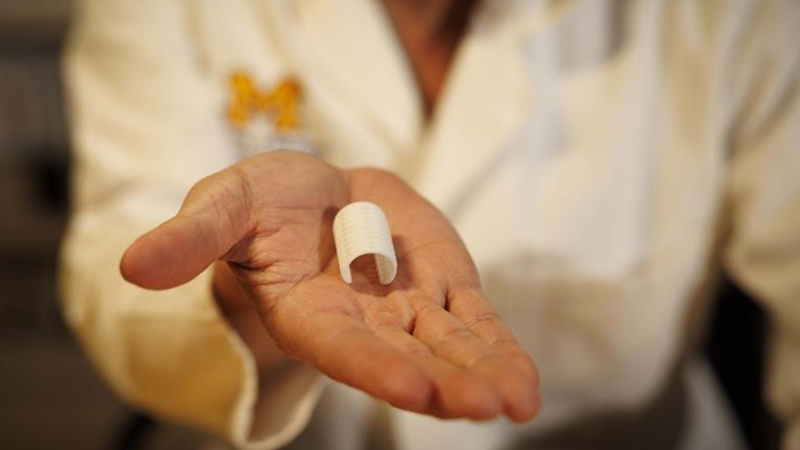
The Michigan Institute for Clinical and Health Research (MICHR) played a key role in a new clinical trial that will allow researchers to study 3D-printed bioresorbable devices aimed at treating children with a rare and life-threatening airway condition, tracheobronchomalacia. The trial, launched by Michigan Medicine and Materialise, marks a crucial step towards full Food and Drug Administration (FDA) approval for the innovative devices designed to support the airways of infants with the severest forms of the disease.
With assistance from MICHR, Mott otolaryngology surgeon Glenn Green, M.D. and colleagues were able to obtain emergency approval to use the bioresorbable scaffolding through working with the FDA, Institutional Review Board and hospital administration. It’s since been used in more than 40 children at Mott.
MICHR, Michigan Medicine and Materialise have worked together to obtain approval for the 3D printed bioresorbable devices to be utilized in a clinical trial. The trial is the next step towards FDA approval to treat children with the life-threatening condition.