CTR-Launch Provides Free Protocol Writing Support
Clinical trial protocols have become increasingly intricate, with a surge in trials utilizing novel designs and continuous expansion in the volume and scope of research regulation. High-quality protocols are more likely to meet enrollment goals, address primary aims, and incorporate operational efficiencies. They can also be easier for less-experienced staff to implement. However, protocol development is time-consuming, and few investigators possess adequate training and experience. While centralized protocol development services can be highly effective, they are often cost-prohibitive. The NIH-funded CTSA Clinical and Translational Research-Launch (CTR-Launch) trial addresses these challenges by testing a novel concept – provision of FREE protocol writing support by a protocol writer to achieve high-quality protocols for investigator-initiated trials (IITs).
Study Population
The study is open to investigator-initiated single-center, non-oncology trials involving protocols reviewed by IRBMED, with the Principal Investigator (PI) agreeing to trial randomization. Interested PIs should read the information provided on this page and email our team to learn more about the study and determine if it’s a good fit for their trial.
Study Design
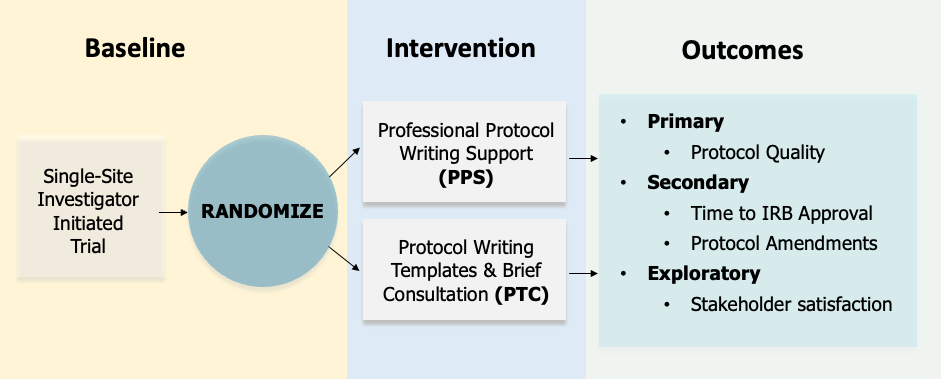
This is a randomized controlled trial that will have a sample size of 134 trials enrolled over 4.5 years comparing Professional Protocol-Writing Support (PPS) with Protocol-Writing Templates & Brief Consultation (PTC).
The interventions for PPS will entail:
- Protocol writer meets with PI/study team to discuss task distributions, deliverables and timeline
- Protocol writer prepares draft protocol and revises as needed
- Final protocol to PI for sign-off and submission to IRB
- Total 40 hours of protocol writer time over 12 weeks from receipt of protocol summary
- 1-time extension of protocol writing support subject to approval
The interventions for PTC will entail:
- One-hour consultation visit with protocol writer (may be extended to 1.5 hours if needed)
- Review of sections/topics in protocol template relevant to the trial
- Up to 30 min of protocol writer time to address questions from research team
PIs in both groups will provide protocol summary to protocol writers after agreeing to include their IIT for randomization into CTR-Launch. The primary endpoint – protocol quality will be assessed by an expert panel using a protocol quality rating tool.

Potential Benefits and Harms of Trial Participation
Potential benefits of this free protocol writing support include:
- Saving time because the protocol writer will write and edit sections of the protocol
- Shorter time to study execution because higher-quality protocols mean fewer questions or issues from IRBMED
- Meeting enrollment goals because the protocol is clearer to study staff and has clearer inclusion/exclusion criteria
Potential harms include the risk of slowing down the process and lowering protocol quality due to a lack of content expertise by protocol writers.
Enrollment
If you’re interested in enrolling in the trial, please email [email protected].